A Message on COVID-19 from WorkSTEPS Chief Medical Officer Dr. Ben Hoffman
COVID-19 Long-Haulers and RTW Decisions
A couple of weeks ago, Bill woke up with a cough and a fever of over 101. He called in sick and quickly got tested for COVID-19. The test came back positive. Today Bill called his supervisor and told her that – although he got pretty sick, the symptoms weren’t bad enough to go to the hospital. He hasn’t had a fever for a couple of days and, while still experiencing a dry hacking cough, “brain fog” and mild fatigue, he wants to return to work.
Two questions: 1) Should Bill return to work? and 2) If Bill does return, how do you address the fear other employees may have in working with Bill?
Long-Haulers
Bill is what has become known as a COVID-19 long-hauler – someone who suffers effects of COVID-19 sickness for weeks and even months after they first experience symptoms. Information from a recent JAMA article on long-haulers includes:
- About 10% of COVID-19 patients suffer prolonged symptoms.
- While long-haulers exhibit symptoms of COVID-19, they do not shed the virus, so they are not contagious.
- Many long-haulers suffered mild or moderate disease that either did not require hospitalization at all, or only a brief period of hospitalization. Click here to read stories from long-hauler COVID-19 patients.
- Protracted symptoms are wide-ranging, but the most common are fatigue, chills and sweats, body aches, headaches, brain fog, and gastrointestinal issues.
- Little is currently known about what causes symptoms to persist or how to help long-haulers recover more fully.
Returning Long-Haulers to Work
First, it’s worth noting that 80 to 90 percent of employees who get sick with COVID-19 will suffer mild symptoms of the disease, and their symptoms will not persist after they recover. Based on CDC guidance, these employees can return to work ten days after symptom onset, providing they are fever-free for 24 hours without the use of fever-reducing medicines.
For employees who suffer mild or more severe disease, and whose symptoms persist after they have otherwise recovered (a.k.a. Long-Haulers), we recommend confirmatory testing (preferably an antigen test) and a clinical consult. The test is recommended to rule out ongoing infection, and a clinical consult will assess symptoms and inform decision-making for a safe return to work. For example, a clinical consult may reveal fatigue that could limit an employee’s capacity to safely drive or operate heavy machinery.
Educating and Assuring Other Employees
Back to Bill. Consideration needs to be given to the employees who work with him and how they will feel when Bill appears fatigued, coughs, or starts to sweat for no apparent reason. They will likely know Bill has had COVID-19 and, understandably, may feel unsafe working around him.
Having easy-to-understand, evidence-based and transparent procedures for returning employees to work is key to alleviating such fears. Employees don’t need to know any personal details about Bill’s health, but it is important that they know that Bill would not be at work without meeting certain requirements. Further, employees can be educated about long-haulers in general – that some people will experience symptoms long after they can no longer transmit the virus to others.
In today’s difficult communications environment, general messaging on these points is necessary but likely insufficient. Plan to deliver information more personally in team meetings and one-on-one if necessary.
The scientific community knows a lot more about COVID-19 today than it just a few short months ago, but there is much more to be learned about ongoing impacts of the disease on some of its victims. It is among the many reasons that – especially as the pandemic surges again – employers need to remain vigilant in not only preventing COVID-19, but in dealing with the many impacts of the disease on employee health, wellbeing, productivity, safety and morale.
COVID-19 is surging again. If your company is looking for an experienced and reliable partner to help navigate COVID-19 challenges, consider WorkSTEPS. More information about our COVID-19 employer services, including testing support and clinical consultation, can be found here.
Ben Hoffman, MD, MPH
Chief Medical Officer, WorkSTEPS
For more from Dr. Hoffman, connect with him on LinkedIn.
Medical:
Global: On Tuesday, the pandemic global case total topped 51 million cases. Disease trend has steepened in parts of Europe. Denmark is rethinking a mandate to cull a large mink population following the identification of mink variant of SARS-CoV-2 viruses called “Cluster 5” that was passed from mink to humans. As a second spike continues in many European countries, hospitalizations and deaths are also on the rise.
National: The US daily COVID-19 total continues to top 100,000 this week. The 7-day average of daily cases was 108,694 on Tuesday, which is a 37% increase from the week before. The Midwest region is experiencing a surge in cases, with North Dakota, South Dakota, Wisconsin, Iowa, Montana and Minnesota all seeing increasing cases. North Dakota hospitals are at 100% capacity and announced that COVID-positive nurses can stay at work.
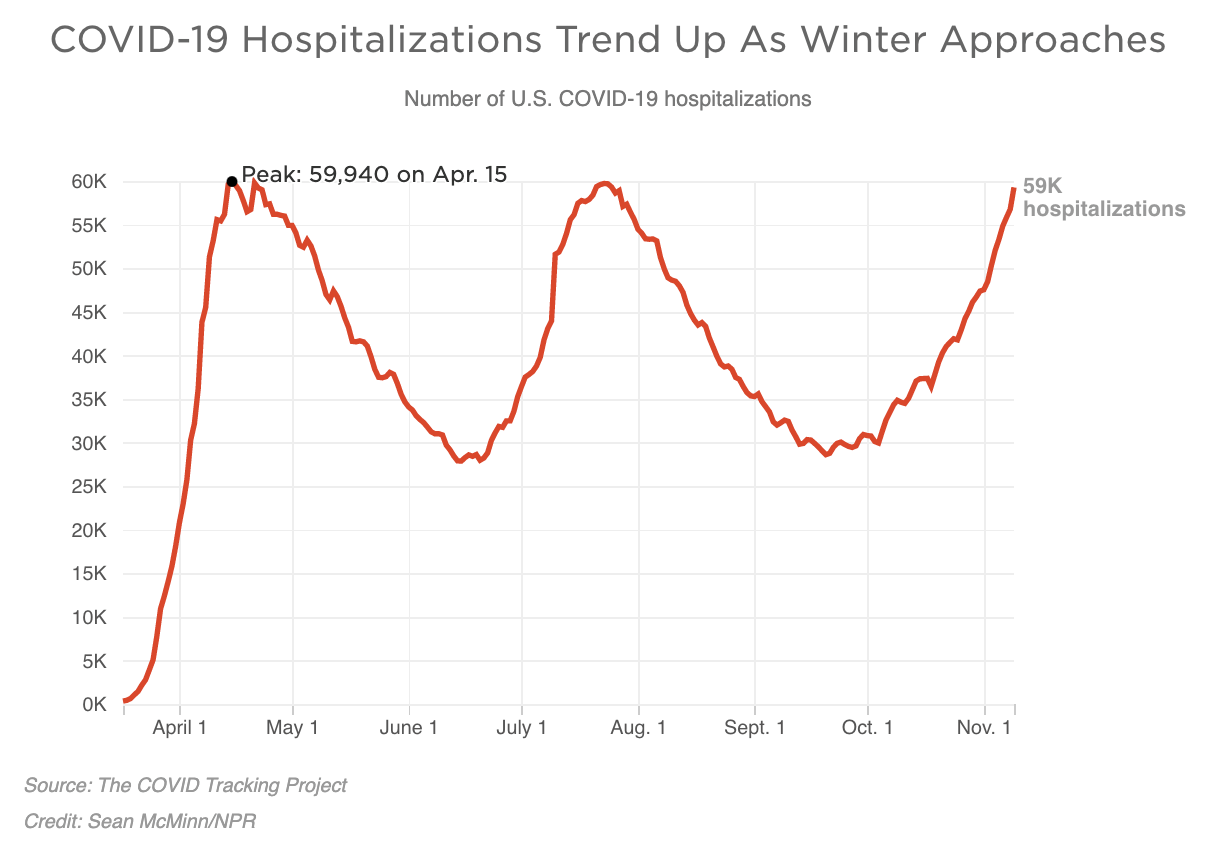
COVID hospitalizations surging: On Tuesday 56,000 people across the nation were hospitalized for coronavirus. 20 states recorded new highs in hospitalizations, while 5 recorded new daily highs for deaths.
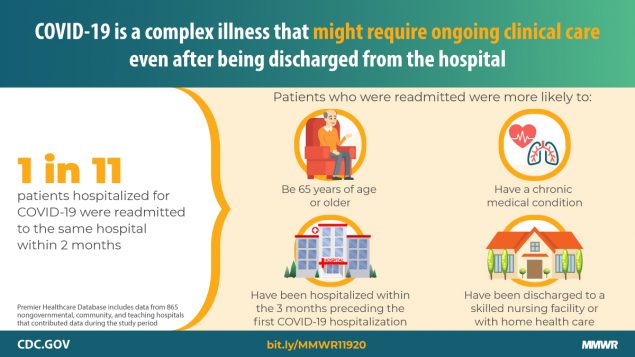
9% of COVID-19 patients readmitted: On Monday, a study published by the CDC’s MMWR Report from showed that, of 106,543 patients who survived after being admitted for COVID-19 from March through July, 9,504 (8.9%) were readmitted to the same hospital within 2 months of discharge. They found that 1,667 (1.6%) of patients recorded more than a single readmission during this time. Of the readmissions, they found that readmissions occurred more often among patients discharged to a skilled nursing facility (15% return rate]) or home health organization support (12%) as opposed to those who were discharged to home or self-care (7%). The most common cause for readmission was an infectious or parasitic illness such as COVID-19 (45%), but 11% were admitted for circulatory diseases and 7% reported digestive diseases.
Severity and Disability in COVID-19 strokes: Last week, researchers published findings that COVID-19 associated strokes are more severe and more likely to result in disability or death than non-COVID strokes are. The researchers reviewed evidence from 86 people who had a stroke in England or Scotland and had COVID-19 at stroke onset, between March and July this year, and compared them to 1,384 stroke cases during the same period in people who did not have any evidence of COVID-19. The researchers found that stroke patients who also had COVID-19 were only half as likely to leave hospital without any disability compared to those without COVID-19.
Review of infective dose, routes of transmission, and outcome of COVID-19 caused by the SARS-COV2 virus: comparison with other respiratory viruses: Exhaled breath of symptomatic patients with influenza can transmit an estimated 33 particles per minute in aerosol. Twenty minutes of exposure would be required for the exposure to the median infective dose of H1N1 subtype. Similarly, almost 25 particle per minute (630 particles in 25 min) in aerosol were required to cause COVID-19 infection in hACE2 mice. Exposure for a similar period to SARS-CoV-2 exhaled in normal breathing of infected patients could lead to the inhaling of our estimated infective dose 300 particles of SARS-CoV-19 by aerosol, thus complementing infection by fomites and droplets. However, further studies are warranted to examine infective dose by the aerosol route and its correlation with COVID-19 severity and immune response both in animals through experiments and humans through observation.
Stability of SARS-CoV-2 on Produce following a Low-Dose Aerosol Exposure: Is grocery store produce a risk? No: Researchers modeled the stability of SARS-CoV-2 on apples, tomatoes, and jalapeno peppers at two temperatures following a low-dose aerosol exposure designed to simulate standard grocery store environments. Samples were collected at 1, 4, 8, and 24 hours. Infectious virus was not recovered postexposure.
When Coffee Smells Like Gasoline: COVID Isn’t Just Stealing Senses — It May be Warping Them: Accounts of parosmia, an odor distortion making things smell different or unpleasant, and phantosmia, which causes people to smell scents that aren’t there, have flooded social media platforms in recent months.
Aspirin to be Investigated as a Possible Treatment for COVID-19 in the RECOVERY Trial (RECOVERY Trial): Aspirin will be investigated in the world’s largest clinical trial of treatments for patients hospitalized with COVID-19. Patients with COVID-19 are at higher risk of blood clots forming in their blood vessels. Platelets, small cell fragments in the blood that stop bleeding, seem to be hyperreactive in COVID-19 and may be involved in the clotting complications. Since aspirin is an antiplatelet agent, it may reduce the risk of blood clots in patients with COVID-19. It is anticipated that at least 2,000 patients will be randomly allocated to receive aspirin 150 mg daily plus usual standard-of-care, and results will be compared with at least 2,000 patients who receive standard-of-care on its own.
Mitigation/Suppression:
FDA clears Eli Lilly coronavirus drug: The FDA approved Eli Lilly’s monoclonal antibody drug ‘bamlanivimab’ for emergency use. Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful antigens such as viruses. The drug has been cleared for treating mild-to-moderate cases of COVID-19 in patients who are over the age of 12 years. In clinical trials, the Eli Lilly drug reduced hospitalizations or ER visits in patients at high risk for disease progression.
Clinicians question the dosing of Eli Lilly’s newly authorized COVID-19 treatment: The FDA on Monday gave emergency use authorization to Eli Lilly’s monoclonal antibody treatment for COVID-19, but the decision has left physicians confused about the best dosage for patients. The agency gave the green light to a 700-milligram dose of bamlanivimab in patients older than 12 who have serious infection but haven’t yet been hospitalized. But data released in September showed that a 2,800-milligram dose was beneficial, compared to two other doses of 700 and 7,000 milligrams. Even then, the finding seemed like a fluke because most patients, even those receiving a placebo, cleared the virus after 11 days, anyway. Experts are now speculating whether the government opted for the 700-milligram dose because the drug is in short supply.
Vaccine Race: Early readout of the Phase III clinical trial of Pfizer’s mRNA vaccine trial results after 94 symptomatic COVID19 cases is very positive (90% effectiveness). The full trial will require 164 additional cases of COVID19 completing the trial. It is reasonable to expect Pfizer will get their full symptomatic COVID19 caseload within the next 2-3 weeks. The estimate of the efficacy of the vaccine could change as the study is completed but is likely to be in the range of 85-95% effective. The Pfizer vaccine requires two doses about 28 days apart. The FDA requires observation on trial participants for two months after immunization to detect possible early serious side effects. Pfizer is advancing its vaccine manufacturing programs and expected to be able to provide 100M doses by March 2021, enough for 50M people. The vaccine will cost about $20 per dose, with two doses required, but the U.S. government acquired the initial doses and expects to provide them to the US public for free, although the health care providers who give the dose may have a fee. Moderna has a similar mRNA vaccine is closely on the tail of Pfizer and will release their trial results soon. The vaccine will be distributed in four phases, with health-care workers, the elderly and people with underlying health conditions getting vaccinated first. Essential workers, teachers and people in homeless shelters as well as people in prisons would be next on the list, followed by children and young adults. That timing from the first cases of COVID19 in January 2020 to effective and safe immunization in December 2020 will be a major biomedical advance. Sources 1, 2, 3
FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection: The US Food and Drug Administration authorized the first serology test that detects neutralizing antibodies from recent or prior SARS-CoV-2 infection, which are antibodies that bind to a specific part of a pathogen and have been observed in a laboratory setting to decrease SARS-CoV-2 viral infection of cells. The FDA issued an emergency use authorization (EUA) for the cPass SARS-CoV-2 Neutralization Antibody Detection Kit, which specifically detects this type of antibody.
Corporate:

COVID-19 risk, socioeconomic differences in telework access: Last week CDC published findings showing the ability to telework reduced the risk of contracting COVID-19.The multistate study examined 314 symptomatic adults who sought care for coronavirus at outpatient testing or health centers from July 1 to July 29, of whom 153 (49%) were SARS-CoV-2–positive. The percentage who reported teleworking full or part-time were significantly lower among infected patients (35%) than among control participants (53%). Researchers concluded that providing the option to work from home when possible, is an important consideration for reducing the risk for SARS-CoV-2 infection. In industries where telework options are not available; worker safety measures should continue to be scaled up to reduce possible worksite exposures.
Risk of severe COVID-19 among workers and their household members: A study yesterday found that employment-related exposure to SARS-CoV-2 endangers workers and their household members; with 46% of at-risk adults living with essential workers, or they themselves are essential employees. Of the 157.3 million workers studied, 112.4 million (71.5%) were essential, and, of these, only 31.2 million could WAH. Among all adults, 49.7% (123.2 million of 248.0 million) were at increased risk of severe COVID-19. Incorporating household members, 46.1% (56.7 million) increased-risk adults either lived with or were themselves essential employees who could not WAH. Researchers concluded that policy makers seeking to make efficient and equitable decisions about the economy and about vaccine distribution should consider the health risks of workers and also of those with whom they live.
The Work From Home WorkSTEPS Medical Team:

Ben Hoffman, MD, MPH
Chief Medical Officer

Tony Nigliazzo, MD
Medical Director

Loraine Kanyare, MSN, MPH, RN
Director of Case Management

Robert L. Levitin, MD
Physician Consultant

Lynda Phillips, LVN
Nurse Case Manager

Codey Church, LVN
Nurse Case Manager

Kerry Womack, LVN
Nurse Case Manager

Chuck Reynolds
Strategic Communications Consultant
This Guidance (“Guidance”) is provided for informational and educational purposes only. It is not intended as Legal Advice or Medical Advice. Adherence to any recommendations included in this Guidance will not ensure successful diagnosis or treatment in every situation. Furthermore, the recommendations contained in this Guidance should not be interpreted as setting a standard of care or be deemed inclusive of all proper methods of care nor exclusive of other methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient, and the known variability and biological behavior of the medical condition. Similarly, this Guidance is based on current advice, comments, and guidance from the EEOC, CDC and the CMS made publicly available. The ultimate judgement regarding the propriety of any specific employment action must be made by the company and attorney in light of all of the circumstances presented by the company, state and federal rules existing at the time and the then current state of the National Pandemic. This Guidance and its conclusions and recommendations reflect the best available information at the time the Guidance was prepared. The results of future studies or changes in rules, regulations or laws may require revisions to the recommendations in this Guidance to reflect new data. WorkSTEPS does not warrant the accuracy or completeness of the Guidance and assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this Guidance or for any errors or omissions.




